#10 - Isocitrate Dehydrogenase - a mother's day story
How a single gene weaves our stories together and an ode to mothers. This is a long one and a little scientifically jargony (with apologies).
Everything is waiting for you
Your great mistake is to act the drama as if you were alone. As if life were a progressive and cunning crime with no witness to the tiny hidden transgressions. To feel abandoned is to deny the intimacy of your surroundings. Surely, even you, at times, have felt the grand array; the swelling presence, and the chorus, crowding out your solo voice. You must note the way the soap dish enables you, or the window latch grants you freedom. Alertness is the hidden discipline of familiarity. The stairs are your mentor of things to come, the doors have always been there to frighten you and invite you, and the tiny speaker in the phone is your dream-ladder to divinity. Put down the weight of your aloneness and ease into the conversation. The kettle is singing even as it pours you a drink, the cooking pots have left their arrogant aloofness and seen the good in you at last. All the birds and creatures of the world are unutterably themselves. Everything is waiting for you.
Our lives are full of synchronicities, congruences that seem aleatory and impossible to weave together. Perhaps they are merely the consequences of the stories we regale ourselves with, to sanctify our need to belong, like Plato’s halves yearning for one another. Perhaps not and something deeper enmeshes us all. Either way, I choose to view these occurrences as gifts, delicious reminders that we exist, never, in vacuum; the ties that bind leaving the residue of an ineffable, unrecognizable and prosopagnostic awe.
I first learned of the gene, IDH, after Kim’s leukemia first relapsed in July 2013. She had been in remission for about 17 months when the leukemia returned with new mutations. The original harbored genetic errors including Trisomy 8 with TP53 and NPM1 mutations. The relapse gave rise to a new set, IDH1, a possible DNMT3A and FLT3-TKD, all identified through a Foundation One panel, and a worse prognosis.
What is IDH - isocitrate dehydrogenase
Normally IDH plays a crucial role in the citric acid cycle (sometimes referred to as the Krebs’ cycle), a key pathway in the cellular production of energy in the mitochondria. Citrate is important for cells to function and when improperly regulated can lead to various challenging neuropathologies including epilepsy associated with SLC13A5 mutations. IDH catalyzes the dehydrogenation of isocitrate to a-ketoglutarate (aKG) as part of this pathway, a seemingly simple and innocuous chemical reaction. When mutated, instead of making aKG, IDH increases the production of a closely related molecule called D-2-hydroxyglutarate (D-2HG, R-2HG, or 2HG for short). It turns out that 2HG is highly leukemogenic and causes cells to arrest their development and reside in an immature blast-like state. These blasts no longer respond to cytokine signals nor do they mature into functional myeloid cells, including red blood cells that we need to “breathe”, and instead crowd out other normal cells, causing the clinical disease of AML. Mutated IDH (mIDH) is not just implicated in AML, but can be associated with other cancers including cholangiocarcinoma (bile ducts), T-cell leukemias and low grade gliomas (brain) while 2HG itself is associated with colorectal cancer, in the absence of mIDH.
2HG competitively inhibits aKG from binding to cognate enzymes such as TET2 and HPH to drive multiple effects that lead to the onset of AML:
altered epigenetic modifications and corresponding gene expression including upregulation of oncogenes
persistent hypoxia, which can alter and sustain tumor metabolism
Direct interaction with T-cells leading to immunosuppression. Interestingly T-cells make (S-)2HG at low levels as part of the resolution phase of inflammation and establishment of immune memory.
DNA instability - acquisition of new mutations that help tumor escape and aid immune evasion
Though it remains unclear what the prognostic implications of acquiring these IDH mutations are, these mechanistic alterations in cellular metabolism, gene expression and tumor immune escape do not bode well for extended remission in an already difficult to treat cancer, such as AML.
Agios, Chris and AG-120
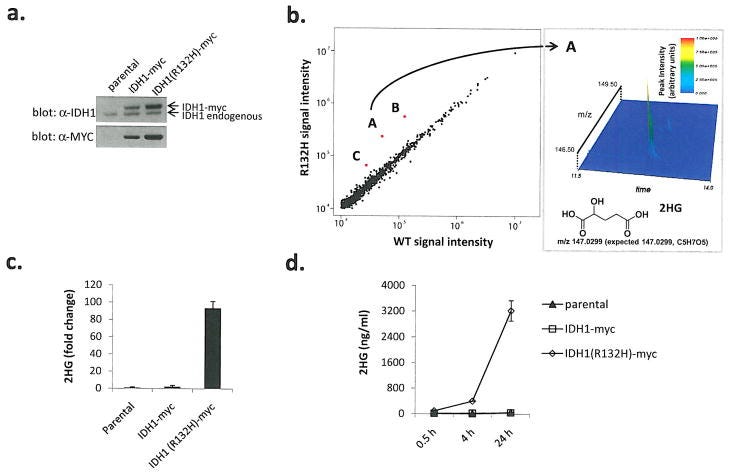
Little was known about IDH1 mutations when Kim relapsed. The first paper identifying the mutations was only published in 2008. But a new biopharma startup Agios1 sprouted from the folds of Third Rock Ventures in 2008 to develop new drugs to target cancer metabolism, including programs that were aimed at inhibiting mIDH1 and mIDH2. Little did I know, that in those early days of Agios’ screening using mass spectroscopy (MS), Chris Emig was consulting for Agios. He now tells me of sitting at the meeting when data were presented, very clearly showing a dramatic MS signature for a metabolite, eventually identified as 2HG, associated with IDH1 R132 mutations, as ultimately published and shown below:
The team at Agios, including Chris, showed that the IDH1 R132H mutant cells show almost a two order of magnitude increase in 2HG production (subpanel c).
Long before I ever met Chris, before we collaborated on CAR discovery or discussed ME-CFS or nerded out on tryptophan biology, he was, in 2009, actively working on the science around IDH1 mutations. We would only meet years later in 2016, well after Kim had died, and I can’t help but think that he was playing a role at Agios, contributing his energy and enthusiasm towards understanding a new mechanism in cancer metabolism that would play a life altering role in Kim’s and my life.
After Kim’s relapse from her BMT in January 2014, we scrambled to find alternative options for her. She was now shunted into 3rd line relapse/refractory AML treatments with few options left. This is usually the point when oncologists give up; her Stanford doctor, Bruno Medeiros, who is a wonderful heme-onc, did his best to mask the certainty with which he had seen in so many cases of AML, of the inevitable disease course towards death. But Kim was obstinate and determined. She worked tirelessly in school and her career and aimed her same resolve towards extending her time with her two young children. Bruno told us she was no longer eligible for another BMT, nor could her heart take much more cardiovascular damage from the anthracycline chemotherapy. Based on the genetic mutations of her tumor, we investigated the possible use of DNMNT3A inhibitors such as azacytidine and decitabine. Bob Nelsen, of ARCH Venture Partners, also worked to find alternatives, pointing us at Phil Greenberg’s HLA-A2 T-cell therapy. He ultimately introduced us to David Schenkein, then CEO of Agios, who directed us to the soon to be open, clinical trial at Memorial Sloan Kettering (MSKCC) for Agios’ drug AG-120 (ivosidenib) targeting mIDH1. Out of all available options, Kim liked the approach that Agios was taking best and reached out to trial’s principal investigator, Dr. Eytan Stein in January 2014 to discuss the possibility of moving to New York to be a part of the trial. But by February, Kim developed an infection, sepsis and ultimately died in March 2014, narrowly missing out on the opening of the trial and potentially being the first AML patient treated, as seen in the timeline below.
History of IDH related therapeutic development.
Eight months later, I attended ASH 2014, and saw Dr. Stein’s talk, reporting on interim phase 1 results of AG-120, embalmed in an intermingled feeling of hope and grief. Just six months after the trial started, MSKCC reported that roughly half of patients showed some positive response on ivosidenib as a single agent drug, with few toxic side effects. These were characteristics of a good drug and Kim would’ve been glad to know that she had picked the right path for herself, and perhaps would somehow help nudge the drug forward to future patients.
Mechanism of Differentiation and Confuscian right
AG-120 is an allosteric inhibitor, which means that it doesn’t directly block the target enzyme’s active site, but instead, binds elsewhere on mIDH1 to alter what chemistry it does. Once in the leukemic cell, AG-120 limits production and reduces the levels of 2HG which unlocks the leukemic blasts from their static state and pushes the reset button so they can resume differentiation. Rather than eliminate the leukemic cells, the way standard chemotherapy does, AG-120 encouraged leukemia to become unstuck and progress towards a mature, normal functioning cell fate.
The mechanism and results of AG-120 were reminiscent of the treatment of another leukemia subtype, APML. Prior to the 1990s, APML was an effective death sentence with very few durable remissions and fewer than two thirds of patients, surviving past five years. Two Chinese researchers, Zhen-Yi Wang and Zhu Chen, working on APML took inspiration from a quote from Confucius:
“If you use laws to direct the people, and punishments to control them, they will merely try to evade the punishments, and will have no sense of shame. But if by virtue you guide them, and by the rites you control them, there will be a sense of shame and of right.”
They built on some previous work that gave patients high dose Vitamin A (or ATRA, all trans retinoic acid) and combined it with Arsenic Trioxide (ATO), which had roots in Eastern medicine. Remarkably, as they tested this cocktail, initially in China and across the globe, soon thereafter as other researchers adopted the approach, they saw APML remission and 5-year cure rates climb to north of 90%. This occurred in a time when medicine from China was regarded with skepticism. The ATRA/ATO story is a wonderful example of Anyone Can Cook, that great art, great science and hope can come from anywhere.
AG-120, and it’s cousin AG-221 which targeted mIDH2, seemed to be tackling leukemia in a similar fashion. Building on the story of the development of ATRA/ATO for APML, a paradigmatic shift from waging war on cancer to re-educating cancer, the results from two new drugs reiterated that this was possible. Guidance, and not punishment or control, could lead to the right pathway. There were now multiple empirical data that validated the science around coaxing cancerous bullies back towards being well behaved adult citizens. An aside: as a strategy, if this can be done to cancer, some of the worst actors in biology, might this serve as inspiration for us to think about the poor actors in our organizations and societies? Are positive cultures, values and missions what we need to re-educate? This metaphor will be explored in a future post.
Too good to be true
By late 2014, Gus and I had started kicking around ideas to work together against cancer and by 2015, we had started Chimera, along with Stanford professor Christina Smolke. I had stopped actively following the progress on Agios’ IDH inhibitors (they were working quite well in clinical trials at the time but data readouts are few and far between). Gus and I hired Anna Mueller as our first employee (now Dr. Mueller), invented F-it! Thursdays and ate South San Francisco dim sum and burritos endlessly. My VW Golf was parked at an expired meter on Grand Avenue in South San Francisco for three days, because Anna had lost my keys after, ahem, innovating at the Standby. Chimera was bursting with fun and energy and we lived life fully, given our acute awareness of how tentative it was. At times Gus talked of a Damoclean sword of relapse hanging over his head and other times we would drive by a funereal procession in Colma and both weep into our tears. But on the whole we felt privileged to work on the problems we wanted to and with each other.
But by October of 2016, Gus learned his wife, Dianna “Charlie” Lester Zeiner, was diagnosed with a stage 2 astrocytoma, a low grade glioma brain tumor. She had been noticing some changes in her vision and what was supposed to be a precautionary MRI, shockingly revealed the cancer. Suddenly Dianna, once the stolid caretaker for Gus, became the patient, marking both Zeiners as unthinkably unlucky.
Dianna is ebullient, a white hot point source of energy, convivially brightening her world. She is also a scientist (Gus will say that she is way better at the bench than he is, which is saying a lot), having spent decades working in drug development at Amgen. She was the first to clone the calcitonin gene related peptide (CGRP) and its receptor at Amgen2, the target of the migraine drug, Aimovig. She is also a native Texan who does not take guff, whether it be from her husband, children, a boss or cancer. Like Gus, as described at Hump Meeting, she courageously embraced her own future, not without trepidation, finding options at Stanford and UCSF, including the incredible neurosurgeon Melanie Hayden. She underwent cranial surgery, had her primary tumor removed and then was subjected to periodic head MRIs to monitor for relapse. Her tumor was sent out for sequencing and, unbelievably, but expectedly, came back as having mIDH1.
Gliomas are littered with IDH mutations as these were where the metabolic mutations were first observed. Melanie is an excellent surgeon and Dianna’s follow up imaging showed clear margins, no evidence of cancer. Dianna, understanding the biology of temozolomide, the standard adjunct alkylating chemotherapeutic used in treating operable brain tumors, rejected her neuro-oncologist’s3 recommendation of shuffling the genetic deck of her tumor and opted for a monitor and observe strategy. The Zeiner family would, once again together, have to agonizingly wait and see.
In the ensuing years, she lived her life vibrantly and purposefully. She made a choice to leave Amgen and joined us at Chimera to tackle cancer. She engineered massively high throughput experiments, one example being our luciferase-based cell killing tests, which she coined the matador assay. We witnessed and felt the full force of her being as she greeted each day with vigor. It must have been Sisyphean labor though, juggling concerns over her own health, dealing with side effects from the surgery a nd worrying over the health of her husband and, perhaps most devastatingly, the emotional impact of twinned parental cancer diagnoses, on her sons’, Trey and Zane. Monthly appointments and MRI followups demarcated the terror of relapse. She bore these burdens with stoic strength. As with all human beings she would falter at times but always responded with dignity and courage.
Dianna (on the right) here with Rachel, rocking some Chimera unicorn vibes.
Meanwhile back at Agios
While Dianna was meandering through the foreign lands of her diagnosis, Agios continued to build their map. The drug, AG-120, marched through the next phases of clinical trials. In 2018, Dr. Courtney DiNardo, an MD Anderson heme-onc who specialized in mIDH1/2 AML disease, and co-authors (including Dr. Eytan Stein) published a New England Journal of Medicine paper reporting on the safe and efficacious use of ivosidenib in mIDH1. These data led to the FDA approving the drug, now named Tibsovo, as a first-line treatment in 2019 for AML. In the five years since Kim spoke with Dr. Stein, AG-120 made it over the regulatory finish line on the back of data showing significant benefit, with a better toxicity profile, for some 10-20% of patients with AML. Agios had already earned an FDA approval for AG-221, for mIDH2, in 2017, and together these two drugs represented a quietly luminous new dawn for patients through the genetic targeting of cancer metabolism.
Santa Monica High School (SAMOHI) Interlude
With the specter of Dianna’s tumor looming, Gus got to work. His interest was rekindled in projects like Delta Notch Like Ligand 3 (DLL3), which becomes an actionable target expressed on the surface of low grade gliomas, a potential CAR-T target. He dug into the biology around IDH in gliomas, noting the confusing contrast of the positive prognostic value of mIDH1 with the negative role that 2HG plays in suppressing T-cell immunity. Consistent with our modus operandi, I would often dig into the literature alongside him, often sharing late Saturday evenings on Slack to work through research papers.
Taking aim at the intersection of immunity, mIDH1 and gliomas, I stumbled across a 2017 article in Genes and Development “Mutant IDH1 regulates the tumor-associated immune system in gliomas” authored by NM Amankulor. Amankulor might have seemed an odd name, but I happened to know a Nduka Amankulor from attending John Adams Middle School and SAMOHI. Sure enough with a little digging, it was indeed my old friend, wrestling teammate and Madrigals singing, operatic tenor, now a neurosurgeon at the University of Pennsylvania. Surprised that my search on mIDH1 would lead our lives to converge years later, I wrote to him, with little expectation that he would have more than little memory of who I was. My message’s excerpt here:
Hey Nduka,
OMG! It's been a long time since SAMO, JAMS, wrestling! I stumbled across a paper of yours on IDH in low grade gliomas on pubmed authored by "N Amankulor" and was like, there is no way that's Nduka, but sure enough here you are.
It's so great to see you and it doesn't surprise me that you are a freaking pediatric neurosurgeon. I was reading up on your paper because I've had a long interest in IDH1 (my wife died of an IDH mut AML) and more recently my best friend's wife just relapsed with her IDH1mut glioma.
…
Love to hear how things are going if you ever get a chance. And if I ever find myself in Philly, I'd love to grab a cheesesteak for you. I'm in the San Francisco Bay Area, if you are ever in the neighborhood and head to SM every so often to visit family.
My best,
Ben
No more than three hours had passed when I received this epistolary response, again in excerpt:
Ben!!!!
Gosh, where do I even start? it’s crazy because I think about you at least once every 6 months. I talk to my kids about how kids from different backgrounds can synergize when they shed the facade of cool and find common values and intellectual interest. For some reason you represent those values for me and I am floored to be hearing from you right now.
I’m so sorry to hear about your wife. It breaks my heart. But, as usual, the Ben that I know has taken a devastating event and turned it into a positive. My dad died of a GBM shortly after I left SM for NYC and that sparked a fire that pushes me till today. IDH malignancies have become the single disease I wish to eradicate. The death of young people from this disease is dismissed by the public at large, and hearing of your wife only inspires me more.
…
Kind of in shock right now to hear from you. It’s a small world, but I always had a feeling we would reconnect at some point. So many similarities: kids of immigrants, open-minded, nerds in a tough environment at JAMS…But our time at JAMS was special and meaningful to me. Let me know if we can catch up in SF.
Wow….
Nduka
What was it about this gene, IDH, that connected all these parts of my lives together - Kim, Dianna, Gus, Chris and now Nduka. How could it be that all of us would be impacted by this together? In the end Nduka and I would get together in SF, when he came for his annual Congress of Neurosurgeons meeting, and reconnect to talk of old times, science and the promise of new treatments to serve those traveling on the horizontal curve of the KM plot.
Dr. Amankulor and Ben in SF during Fleet Week 2022. It had only been about 30 years since we saw each other, united by IDH, circumstance, openness and a mission to help patients.
The reunion unicorn
By 2022, Dianna’s cancer had remained in check for six years, approximately the median time for gliomas to relapse and to progress to glioblastoma multiforme. Her MRI late that year uncovered progression in an inoperable location. Gus’ post to the Chimera Slack channel that day:
“Dianna had her 6 year MRI last week, and I’m sad to report that it showed tumor progression. This is unfortunate news, but it is not unanticipated. Quiescence followed by progression is what grade II, IDH1-mutant gliomas do, and I’m grateful that we had 6 years of treatment-free, progression-free living since her surgery.
As some of you know, her original surgery was at Stanford, but we switched to UCSF for neuro-oncology follow-up at year 2. UCSF is a neuro-onc powerhouse and they have a lot of experience with Tibsovo and Vorasidenib, which are IDH1 inhibitors. Tibsovo is now approved for AML and also drives durable PR and SD in glioma patients. Vorasidenib (a potent combination IDH1/2 targeting inhibitor that is small enough to cross the blood brain barrier) is in phase 3 and the trial is fully enrolled. She is going to start Tibsovo in two weeks, and her oncologist is optimistic.
I’ll keep you posted on how this all plays out. Thanks for bearing with us, and for doing what Chimera does. Because the things we do matter.”
Dianna would receive Tibsovo in an off-label use case. Yes the same drug that Kim uncovered in 2013, whose 2014 Phase 1 trial she just barely missed out on being enrolled in and subsequently approved for AML, was now going to be given to Dianna eight years later. It seemed that the connective fascia that binds our human existence, was resounding harmonically. Dianna’s follow up to Gus’ Slack post:
Hello Chimera family! Know I am upbeat and optimistic about this next phase of my cancer saga. Yes, I am annoyed that my tumor has come back when I was was feeling totally balanced and happy with life. Know I have plenty of energy to fight this and that I have no plans on giving up. For those of you that know me, you are fully aware about how open I can be about such things.
I could hear the faint echoes of Kim’s hopeful voice in Dianna’s, maybe alongside and in sympathy.
Insurance
But there was dissonance. Because ivosidenib is a relatively large molecule, it was considered not particularly useful in a brain tumor setting, with its perceived difficulty to cross the blood-brain barrier. Tibsovo is an expensive drug, costing roughly $30,000 per month. Because Dianna was to receive it under an off-label use case, as Tibsovo is not approved for glioma, a health care plan had the option to deny coverage, leaving the burden of the medical costs to the family. This is indeed what happened when Blue Shield opted out of covering the cost of this potentially life saving drug, despite multiple direct appeals. The Zeiners searched for other patient access programs and clinical trials, but Dianna didn’t qualify for those alternative options. They were left in a crucible forced to choose between financial hardship or Dianna’s chance at life.
In California, where we reside, there is an agency called the Department of Managed Healthcare (DMH), to whom patients can appeal to when payers deny coverage. Previous attempts to overturn coverage decisions regarding Tibsovo had failed three out of four times at the DMH. Gus and Dianna worked tirelessly to craft a scientifically deep and convincing articulation for why Tibsovo should be used in Dianna’s case. Gus drew on his deep molecular biology expertise, his decade working on cancer therapeutics and leaned on guidance from key opinion leaders in oncology. Ultimately they were awarded a narrow decision, 2-1, in favor of overruling Blue Shield’s decision. Dianna would get her drug and it would be covered.
I can only wonder and ache for all the patients who don’t have the resources, expertise and drive that Gus and Dianna have, to be able to convincingly describe their medical need. If you are reading this somehow, and you’re one of those patients who need assistance, please reach out to us. Our mission is to assist all patients on their journey, to the best of our ability.
What’s the story morning glory?
What happens next? Dianna breaks her ankle five minutes into hiking Waimea Canyon in Hawaii, a few months later. And by her year seven MRI, her tumor has regressed. Now Dianna has metal pins in her foot, but Tibsovo worked and continues working for her as the tumor shrinks. The science holds and despite being a mostly logical scientist, I get the feeling that Kim must have helped in some way. And in Dianna, I see the same hope that Kim held for herself.
IDH has bound many of us together: patients, physicians, researchers, families, children, those who have yet to know how they ultimately connect to us. Courtney DiNardo, who is mentioned above a couple times, is the better half of Dr. Andrew DiNardo, an infectious disease doctor at Baylor who spends a third of his year in Eswatini researching and fighting tuberculosis. While TB is of little concern in the West, it still plagues African countries, causing significant numbers of unacceptable deaths. Andrew and I collaborate on some other projects, to be described in future posts, but I find that IDH has also bound me to him and Courtney, two courageous physician-scientists. Who else shall I be braided with, through IDH?
Ultimately this is not the story of some random gene that unites us. More than the biology, the evidence of a human tapestry acts as my guiding light; I, merely amanuensis, record and imagine all of the tiniest, different threads of our individual selves that we impossibly share, interlace with one another to orate a shared story. We hold these in common and they amalgamate us to each other. The story of IDH is one of human connection.
An ode to mothers - the weavers of all our stories
I sought, originally, to write this story as an adulation to mothers and the link between two mothers. I had hoped to get this ready by last Sunday’s Mother’s Day, but could not complete it in time. I had written this to a loved one:
I’m not such a fan of mother’s day in so much that I find it too claustrophobically contained. Mother is a verb, an enduring act of constant creation, shaping our collective future through her nurturing of her children, whether by blood, chance or the necessary of human longing.
What I meant by this are two things:
As my daughter Rilka put to me “Mother’s day should be celebrated every day. Father’s day… once a year is good enough”
Mothers are the ultimate connectors of humanity and creators of our world. They bring us together in impossible ways that we can barely understand but feel intuitively and viscerally. It is the feeling of a literal, constant labor of love.
And in this connection, from Kim to Dianna: I love you. I hope for you that our shared genetics offers you, more than medicine and science, all that a life must: age, ache, joy, love and life. I hope I am ever tied to you and Gus and Ben, our children, and all the rest of the world.
In Chimera’s early days we would share this photo of our families at the end of every presentation, investor pitch or hump meeting - as a reminder of who we were toiling for. Now looking back, it was the picture of two mothers who sought to live, to mother their children, one another, and all of us in the world. In honor of them, this for all who mother.
Where we are now in 2024:
You, we, are ALL still mothering. And thanks to you, we are all doing ok.
Wild Geese
You do not have to be good.
You do not have to walk on your knees
for a hundred miles through the desert repenting.
You only have to let the soft animal of your body
love what it loves.
Tell me about despair, yours, and I will tell you mine.
Meanwhile the world goes on.
Meanwhile the sun and the clear pebbles of the rain
are moving across the landscapes,
over the prairies and the deep trees,
the mountains and the rivers.
Meanwhile the wild geese, high in the clean blue air,
are heading home again.
Whoever you are, no matter how lonely,
the world offers itself to your imagination,
calls to you like the wild geese, harsh and exciting -
over and over announcing your place
in the family of things.
- Mary OliverAddendum
Additional references on IDH
Beyond Brooding on Oncometabolic Havoc in IDH-Mutant Gliomas and AML: Current and Future Therapeutic Strategies4
To learn more about Agios, Jerome Groopman wrote an excellent New Yorker article about Agios’ development of the compound AG-221, which was designed to target mutations in IDH2. Josh Elkington, of Axial, does a nice case study and history of Agios.
Dianna noted that I made an error in my original statement, crediting her to be the first to clone CGRP. Instead, as she pointed out, it was Aiyar Nampo, published here. Thanks for the feedback D and the correction!
When you develop a brain tumor you get receive both surgeon and oncologist
Madala HR, Punganuru SR, Arutla V, Misra S, Thomas TJ, Srivenugopal KS. Beyond Brooding on Oncometabolic Havoc in IDH-Mutant Gliomas and AML: Current and Future Therapeutic Strategies. Cancers (Basel). 2018 Feb 11;10(2):49. doi: 10.3390/cancers10020049. PMID: 29439493; PMCID: PMC5836081.










Ben, I am honored to be part of this story and I marvel at the connections you eloquently described. Kim will always hold a piece of my heart, and it grows even more realizing through your words our connection. I did not know she too was hopeful for AG-120. Long before the drug ever got the name Tibsovo, I was following the promise of a small Boston company whose theme to find “the other side of possible”. This was my mantra and guided me through my own research, hoping to not only give back but also “fight cancer with my pipette”. Though cancer is sad and demotivating, I remember those who influenced my life, their stories giving me strength. Meeting you and Kim, building a friendship through these years, is one of the most special relationships I am honored to have. Bearing witness to this revolutionary change in cancer treatment, as both a scientist and a patient, is one of the highest accolades of my life. The top being a mother.
Thank you for your words, I will cherish this always.
Note to self, as of December 2024, AG-120, AKA tibsovo, has subsequewntly wrecked Dianna's tumor, shrinking it dramatically. Hell to the yeah, D!